Frequently Asked Questions about Human Subjects Research
FAQS
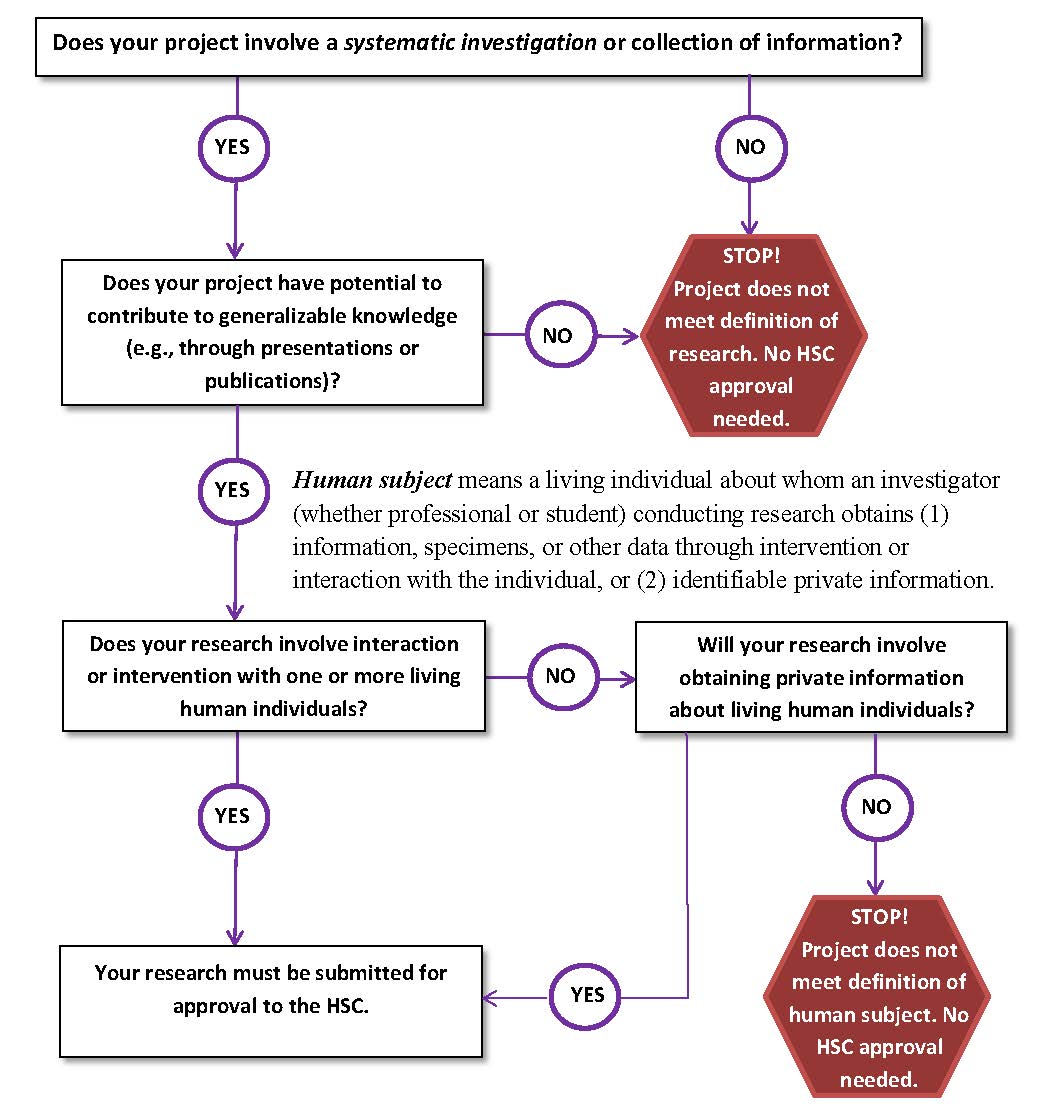
HSC review is required for studies that meet the definitions of “research” and “human subject.”
According to the regulations, research is any systematic investigation designed to develop or contribute to generalizable knowledge. Any activity that meets this broad criterion and that is conducted by UNA faculty, administration, staff, students, and contracted consultants or that uses UNA facilities is research for the purposes of this discussion. It does not matter whether the activity occurs within and as a part (however large or small) of some other activity, such as a demonstration or service program, or whether the research is the whole project.
Regulations define a human subject as a living individual about whom an investigator obtains either (1) data through intervention or interaction with the individual, or (2) identifiable private information. Intervention generally includes physical procedures by which we gather data (e.g., venipuncture) and manipulations of the subject or the subject’s environment for research purposes. Interactions that include communication or interpersonal contact between the investigator and the subject are more common. Private information includes information about behavior that occurs in a context where the individual can reasonably expect no observation or recording. Thus, the individual will have provided the information for specific purposes and can reasonably expect that the information associated with his or her identity will not be made public.
The following chart will help you determine if your study is subject to HSC review:
Publicly available datasets are those that contain data that are available to anyone regardless of occupation, purpose, or affiliation, and have legitimately attained such status; i.e., those individuals who are responsible for posting the dataset had legitimate access to the data and have employed the necessary mechanisms to ensure the privacy and confidentiality of the individuals about whom the data were collected. In general, while the use of such data may meet the regulatory definition of research, the definition of human subjects is not met because data about a living person is not obtained through interaction or intervention, and no private, identifiable information about a living individual is obtained.
The HSC has reviewed the datasets listed below and determined that they meet the criteria for “publicly available datasets.” However, many of the datasets listed have a publicly available component and a restricted use component. The use of data from these UNA HSC-approved public data sets is not considered human subject research as long as the following two criteria are met:
- Research will NOT involve merging any data set so that individuals might be identified.
- Researchers will NOT enhance the public data set with identifiable or potentially identifiable data.
If the two criteria above are met, and the research involves data from a dataset listed below, no UNA HSC review or approval is needed. If both requirements are not met, an HSC review is required.
|
Site of Data |
Data Set |
|
(ATUS) |
|
|
(ICPSR) |
Public-Use Data Files Only – excludes restricted and enclave datasets |
|
|
|
|
|
(NTDB) |
|
(NCES) |
Public-Use Data Files including, but not limited to: |
|
(CDC) |
(BRFSS) National Ambulatory Medical Care Survey (NAMCS) and |
|
|
|
|
(AHRQ) |
|
|
(NSCAW – General Use Files Only) |
|
|
|
|
|
|
|
|
Public-Use Data Files Only – excludes restricted datasets |
Definitions
Exemption
Investigators submitting protocols for studies that qualify for exemption are generally notified within 10 working days or less.
HIPPA
Reporting
Special Classes (Populations) of Subjects
Training
- You need HSC training if you are an employee or student at UNA and you
- are listed as an Investigator or "key personnel" (i.e., engaged in the design, conduct, analysis, or reporting of research) on the protocol or funding application; or screen potential participants and/or obtain informed consent;
- collect, analyze or access the data, or have HSC-related responsibilities.
- are listed as an Investigator or "key personnel" (i.e., engaged in the design, conduct, analysis, or reporting of research) on the protocol or funding application; or screen potential participants and/or obtain informed consent;
- You do not need HSC training if
- are not affiliated with UNA in any way (e.g., student, employee, consultant);
- and work at another institution that has its own IRB.
- are not affiliated with UNA in any way (e.g., student, employee, consultant);
Unanticipated Problems/Adverse Events
- Unanticipated problems are any incident, experience, or outcome that meets all of the following criteria:
- unexpected (in terms of nature, severity, or frequency) given (a) the research procedures that are described in the protocol-related documents, such as the HSC-approved research protocol and informed consent document; and (b) the characteristics of the subject population being studied
- related or possibly related to participation in the research (in this guidance document, possibly related means there is a reasonable possibility that the incident, experience, or outcome may have been caused by the procedures involved in the research); and
- suggests that the research places subjects or others at a greater risk of harm (including physical, psychological, economic, or social harm) than was previously known or recognized.
- unexpected (in terms of nature, severity, or frequency) given (a) the research procedures that are described in the protocol-related documents, such as the HSC-approved research protocol and informed consent document; and (b) the characteristics of the subject population being studied
- Unanticipated problems must be reported to the Director, Office of Sponsored Programs and HSC Chair as soon as practicable but no later than five days after the occurrence. See Unanticipated Problems and Adverse events section of the UNA Human Subject Research Program Policy for guidance and form.
Other FAQs Sources
Do I need HSC approval?
Education and Training
Ethical Principles
FAQs
Policies, Guidelines & Forms
Public Datasets
Regulations
Related Links